a Chinese Field Epidemiology Training Programme, Beijing, China.
b The Global Fund to fight AIDS, Tuberculosis and Malaria, Geneva, Switzerland.
c Zhongshan Municipal Center for Disease Control and Prevention, Zhongshan, China.
d National Immunization Programme, Chinese Center for Disease Control and Prevention, Beijing, China.
Correspondence to Huiming Luo (e-mail: Hmluo@vip.sina.com).
To cite this article:
Wang M et al. Mumps-containing vaccine effectiveness during outbreaks in two schools in Guangdong, China, 2012. Western Pacific Surveillance and Response Journal, 2012, 3 (4):29–32. doi:10.5365/wpsar.2012.3.4.012
Introduction: Mumps-containing vaccine was licensed in the 1990s in China with a single dose administered routinely to children aged 18-24 months since 2008. However, an increase in reported mumps cases during the period 2009 to 2012 casts doubt on the effectiveness of a single-dose mumps vaccination. In March 2012, large numbers of mumps cases in a day-care centre and primary school in Guangdong Province were investigated to estimate the effectiveness of mumps-containing vaccine.
Methods: A mumps case was defined as a case with acute onset of unilateral or bilateral swelling of the parotid gland or other salivary glands. Clinical data were collected among students and staff members in the two schools from 6 February to 3 June 2012. Vaccination history was obtained from immunization certificates. Vaccine effectiveness (VE) was calculated among children in classes that had more than two mumps cases.
Results: The cohort included 369 children from seven classes, four from the day-care centre and three from the primary school. Vaccination certificates available for 347 children showed immunization coverage of 82% (285/347). The overall attack rate was 14.6% (54/369); the VE for a single dose of mumps vaccine was 65% (95% confidence interval [CI]: 19%-85%) when given within three years and 15% (95% CI: -2%-52%) when given three to six years before the outbreak. For two doses of vaccine the VE was 53% (95% CI: -15-80%).
Discussion: A single dose of mumps-containing vaccine was not effective to prevent these outbreaks among preschool and school children. A second dose of mumps-containing vaccine to four to five-year-old children should be considered in China.
Mumps, an acute viral illness characterized by unilateral or bilateral tenderness or swelling of the parotid or other salivary glands, is transmitted through person-to-person contact or by direct contact with respiratory droplets or saliva from an infected person.1 Mumps-containing vaccines are now available globally for the prevention and control of mumps. Since 1990, live attenuated mumps vaccine has been licensed in China, and has been included in national routine immunization programmes since 2008. Children aged 18–24 months routinely receive one dose of measles-mumps-rubella vaccine (MMR) free of charge. However, data from the China Information System for Diseases Control and Prevention showed that the number of reported mumps cases continued to increase, with incidence rates of 22.5 per 100 000 in 2009 and 33.9 per 100 000 in 2011, with children aged five to six years having the highest incidence rate. From 2009 to 2011, the numbers of annually reported mumps outbreaks in China were 466, 265 and 440 respectively, and nearly 75% of the reported outbreaks occurred in preschool centres and primary schools.
In March 2012, two separate mumps outbreaks were reported in a day-care centre and a primary school in Guangdong Province, China. The Chinese Field Epidemiology Training Programme was requested to investigate the two outbreaks. The objectives of the investigation were to establish a retrospective cohort to examine mumps-containing vaccine effectiveness (VE) and to assess whether the length of time between vaccination and subsequent illness were related to vaccine failure.
A mumps case was defined as a case of acute onset of unilateral or bilateral swelling of the parotid gland or other salivary gland in a student or staff member in the two schools from 6 February to 3 June 2012. An outbreak class was defined as a class with more than two mumps cases and was the study population for the data analysis. Mumps cases among vaccinated students were defined as having swelling of the parotid or other salivary glands and having a vaccination history with mumps-containing vaccine before the outbreak.
Case-finding was undertaken from reports from the school doctor and questionnaires completed by parents until the maximum incubation period (25 days) after the onset of the last case; the outbreak was then declared over. The questionnaire included information on any history of mumps before the current outbreak. Vaccination status and timing of vaccination for each student before the outbreak was obtained from immunization certificates. Mumps-containing vaccines included monovalent, bivalent (measles and mumps) and trivalent (measles, mumps and rubella) formulations.
Vaccination coverage before the outbreak was calculated as the proportion of vaccinated students, with students with unknown vaccination status excluded, using the equation: coverage rate = (one dose + two doses)/(no vaccine + one dose + two doses) * 100%. VE for mumps-containing vaccine was estimated using the equation: 1 - relative risk (RR) * 100%, where RR = attack rate of vaccinated students/attack rate of unvaccinated students, as described by Orenstein et al.2 When estimating the effectiveness of one dose, people who had received two doses were excluded from the calculations of attack rates of vaccinated students. Similarly, people who had received one dose were excluded from calculations when estimating the effectiveness of two doses. We evaluated whether time between vaccination and current outbreak was a potential risk factor for vaccine failure among single-dose mumps-containing vaccine recipients by calculating VE for those vaccinated less than three years versus three years and older. Epi Info 3.5.1 was used for data analysis.
The outbreaks were detected and reported by the school doctors to the local center for disease control and prevention on 22 March 2012 for the day-care centre and on 16 April 2012 for the primary school. MMR was provided free of charge to students who did not develop mumps-like symptoms in the day-care centre on 24 March and in the primary school on 17 April 2012.
During the outbreak period, a total of 68 mumps cases were identified in the two schools. Four classes in the day-care centre and three Grade 1 classes in the primary school were identified as outbreak classes comprising 189 and 180 students, respectively. Mixing of students mainly occurred in the classroom.
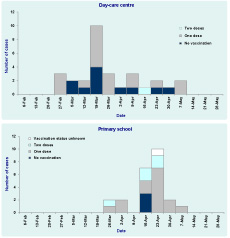
Among the 33 mumps cases at the day-care centre, 30 (91%) were in the four outbreak classes with a mean age of five years (ranges four to six years) and 61% (20/33) were male. In the primary school, 33 students were identified as mumps cases with a mean age of eight years (range: seven to 11 years) and 70% (23/33) were male. Of the 33 student cases, 24 (73%) were from the three outbreak classes. There were two teacher cases. The epidemic curve of the seven outbreak classes (n = 54) shows the number of doses of mumps-containing vaccine that each case received (Figure 1).

Of the seven classes investigated, the vaccination status for 21 children in the primary school and one child in the pre-school centre were unknown since they could not supply vaccination immunization certificates. The coverage rate of the three outbreak classes in the primary school and four outbreak classes in the preschool centre were 90% (143/159) and 76% (142/188), respectively.
Of the seven classes investigated, none of students had a history of mumps before the outbreak. The combined attack rate was 22.6% (14/62) among unvaccinated students, 14.4% (33/229) among vaccinated students with single dose and 10.7% (6/56) among vaccinated students with two doses.
The estimate of VE for a single dose of the mumps-containing vaccine against clinical mumps was 36% (95% confidence interval [CI]: -12%–63%), and 53% (95% CI: -15%–80%) for two doses. Single dose of mumps VE was 65% (95% CI: 19%–85%) within three years after the vaccination, and declined to 15% (95% CI: -52%–52%) in three to six years after the vaccination (Table 1).
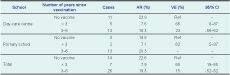
AR – attack rate; VE – vaccine effectiveness; CI – confidence interval.
* Students with unknown vaccination status or two doses of mumps vaccination before the outbreak were excluded.
These two outbreaks of mumps occurred in a day-care centre and primary school in Guangdong Province, China, which had mumps-containing vaccine coverage of 76% and 90%, respectively, before the outbreak. The results demonstrated that the VE of single-dose of mumps vaccine was 65% within three years, and 15% within three to six years. VE must be at least 79%–95% to interrupt mumps community transmission with a coverage rate of 95%.3 Our study suggested that a single dose of mumps-containing vaccine was not effective enough to prevent outbreaks among preschool and schoolchildren.
Three mumps strains are used in China: Jeryl Lynn, RIT 4385 and S79. Both RIT4385 and S79 both were developed from the Jeryl Lynn vaccine strain. In post-license studies, the VE estimate of a single dose and two doses of the Jeryl Lynn mumps-containing vaccine was 79% (range: 62%–91%) and 88% (range: 79%–95%), respectively.1 Two other studies reported a VE for the S79 strain at 86% (95% CI: 77%–92%)4 and 80% (95% CI: 60–90%), respectively.5 The VE estimates in our study were lower than these; however, it is difficult to identify the cause and may be multifaceted due to the case definition, exposure, attack rate, time since vaccination and age of vaccination. Six per cent (22/369) of the students were excluded from analysis since they could not provide immunization certificates, and the attack rate of students among them was 4.5% (1/22). If all of the students with unknown vaccination status had either one dose or two doses of mumps-containing vaccine history, the estimates of VE would be higher, 40% for one dose and 60% for two doses.
Since the clinical manifestation was specific for mumps, we relied on clinical diagnosis and did not ask for laboratory confirmation. The estimates of VE may be imprecise owing to the small number of cases, as reflected by the wide confidence interval. Despite the limitations, to our knowledge the current study was the first rigorous cohort study of outbreaks to estimate mumps VE in China.
Since 2009, reported mumps cases have increased in China, with a large number of mumps outbreaks occurring in preschool centres and primary schools. However, data from the China Information System for Diseases Control and Prevention showed that, in the three provinces (Beijing, Tianjin and Shanghai) that have a two-dose mumps vaccination policy, the reported number of mumps cases have declined sharply since 2009, as have the number of mumps outbreaks (J Liu, Department of National Immunization Programmen, Chinese Center for Disease Control and Prevention, Beijing, China). In our study, those students receiving two doses of mumps-containing vaccine had an estimated VE of 53%, which was higher than that of those receiving a single dose (36%), although this was not significantly different, possibly due to small sample size. This study also suggests that mumps VE may decline three years after vaccination, and previous studies also documented increased risk of developing mumps with increasing time after vaccination.6–8 We recommended that a second dose of mumps-containing vaccine to four- to five-year-old children be considered in China, and this has been communicated to the Ministry of Health.
None declared.
None.