a Department of Laboratory Medicine, National University Hospital, Singapore 119074. Singapore.
b Department of Laboratory Medicine, Tan Tock Seng Hospital, Singapore 308433. Singapore.
Correspondences to Jeanette Teo (e-mail: jeanette_teo@nuhs.edu.sg).
To cite this article:
Teo J et al. Molecular characterization of NDM-1 producing Enterobacteriaceae isolates in Singapore hospitals. Western Pacific Surveillance and Response Journal, 2012, 3(1):19-25. doi:10.5365/wpsar.2011.2.4.010
Objective: In this study, we molecularly characterized 12 NDM-1 producing clinical Enterobacteriaceae (Klebsiella pneumoniae, Escherichia coli, Enterobacter cloacae) isolates that were part of a collection of non-carbapenem susceptible isolates obtained during a one-year period. These isolates were obtained from four local general hospitals in Singapore.
Methods: Polymerase chain reaction (PCR) assays and sequencing was used to determine the presence of β-lactamase encoding genes (bla) including blaNDM-1 and plasmid-mediated quinolone and aminoglycoside resistance determinants. Conjugation experiments were performed to determine the transferability of blaNDM-1. Isolate relatedness was determined by multilocus sequence typing (MLST).
Results: The isolates were completely resistant to the second- and third-generation cephalosporins tested as well as carbapenems. Susceptibility profiling of the isolates indicated that 100% retained susceptibility to tigecycline while 11/12 (91.7%) were susceptible to colistin. The blaNDM-1 gene was encoded on plasmids that were easily transferable. None of the patients had a travel history to countries where NDM-1 has been reported. The isolates appear clonally unrelated with MLST, revealing a diversity of clonal types among the Klebsiella pneumoniae and Escherichia coli isolates.
Conclusion: The ease of NDM-1 plasmid transmissibility may help their dissemination among the Enterobacteriaceae. Although it appears that the isolates are clonally unrelated, epidemiological links cannot be fully excluded without further research.
The discovery of a novel carbapenemase, the New Delhi metallo-β-lactamase-1 (NDM-1), generated much global alarm. These NDM-1 producing isolates gained media notoriety being labelled as superbugs which had the reputation of being impossible to treat. The first carbapenem-resistant NDM-1 isolates characterized in 2009 were Klebsiella pneumoniae and Escherichia coli isolated from a Swedish patient who had sought medical care in New Delhi, India. The strains were resistant to all antibiotics tested except colistin.1 The ease of β-lactamase encoding genes (blaNDM-1) dissemination has become apparent with the worldwide detection of NDM-1 producers.2–6
In this study, we provide the molecular characterization and epidemiology for 12 NDM-1 positive clinical isolates. These isolates were obtained as part of a hospital surveillance programme for carbapenem non-susceptible Enterobacteriaceae.
During the period of August 2010–August 2011, 52 non-duplicate carbapenem non-susceptible clinical isolates from local hospitals were analysed. The isolates were submitted from four hospitals that represented 40% of the general hospitals in Singapore. The 52 isolates comprised the following species: 31 Klebsiella pneumoniae, 13 Escherichia coli, seven Enterobacter cloacae and one Enterobacter aerogenes. Two of these isolates (594 and 693) were obtained from the same patient but from different collection sites (Table 1). The identification and initial susceptibility testing of the isolates was performed with VITEK 2 automated system (bioMérieux Vitek, Inc., Hazelwood, MO, USA). The Etest MBL (bioMérieux, Marcy l’Etoile, France) was used for the phenotypic detection of metallo-β-lactamases.

Note: NEG – PCR assays were negative for the bla genes screened; NUH – National University Hospital, Singapore; PH – Private hospital, ward information was unavailable; ST – Sequence type determined by multilocus sequence typing (MLST); TTSH – Tan Tock Seng Hospital, Singapore; NA – Not available; and ND – Not done.
Carbapenem resistance and resistance to other classes of antibiotics were confirmed by the Etest (bioMérieux) method with minimum inhibitory concentrations (MICs) determined after a 24-hour incubation at 37°C. Susceptibility was defined according to the breakpoints of the European Committee on Antimicrobial Susceptibility Testing. Escherichia coli ATCC 25922 was used as the quality control strain for antimicrobial susceptibility testing.
Plasmids were extracted using the Plasmid Mini Kit (Qiagen, Hilden, Germany). Plasmids were separated on 0.7% Megabase Agarose (Bio-Rad, Hercules, CA, USA) and their sizes estimated using BAC-TrackerTM Super-coiled DNA Ladder (Epicentre, Madison, WI, USA) as a reference. Southern hybridization analysis was performed using DIG DNA Labelling and Detection Kit (Roche Diagnostics, Mannheim, Germany) and digoxigenin-labelled 291 bp blaNDM-1 was used as the probe.
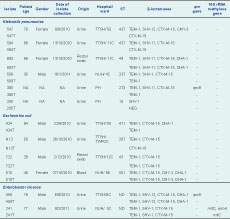
Genomic DNA used for the polymerase chain reaction (PCR) assays was extracted from the isolates using the DNeasy Blood and Tissue Kit (Qiagen). The presence of genes encoding carbapenemases and extended-spectrum β-lactamases was detected using various primers (Table 2). Full-gene sequencing of blaNDM-1 was carried out using NDM-FF and NDM-RR primers (Table 2). This allowed the amplification of the 815 bp NDM-1 gene. In addition to blaNDM-1 detection, bla genes for acquired MBLs (VIM-type, IMP-type and KHM-1), serine carbapenamases (OXA-48, KPC-1, GES-1, -2, -3, -4, -5 and -7) and extended-spectrum β-lactamases (TEM-type, SHV-type, CTX-M-type, DHA-1, CMY-type) were also PCR screened (Table 2). National Collection of Type Cultures strain 13 443 was used as the blaNDM-1 PCR positive control. Plasmid-mediated quinolone (qnr genes) and 16S rRNA methylase aminoglycoside resistance determinants were analysed by PCR (Table 1). The presence of qnrA, qnrB, qnrC, qnrD and qnrS was determined using published screening protocols.10,11 PCR assays were performed using HotStar Taq Plus Master Mix Kit (Qiagen) and setup according to the manufacturer’s instructions. All amplicons were sent for sequencing at a local company (1st BASE, Singapore).
MLST was carried out using the protocol developed by Institut Pasteur (http://www.pasteur.fr/recherche/genopole/PF8/mst/index.html) for Klebsiella pneumoniae isolates. Internal fragments of seven housekeeping genes: rpoB (β-subunit of RNA polymerase); gapA (glyceraldehyde 3-phosphate dehydrogenase); mdh (malate dehydrogenase); pgi (phosphoglucose isomerase); phoE (phosphorine E); infB (translation initiation factor 2); and tonB (periplasmic energy transducer) were amplified and directly sequenced. For Enterobacter coli isolates, MLST was performed using eight housekeeping genes: dinB (DNA polymerase); icdA (isocitrate dehydrogenase); pabB (para-aminobenzoate synthase); polB (RNA polymerase Pol II); putP (proline permease); trpA (tryptophan synthase subunit A); trpB (tryptophan synthase subunit B); uidA (β-glucuronidase). Internal fragments of these genes were amplified and sequenced. The assignment of sequence types was carried out at http://www.pasteur.fr/recherche/genopole/PF8/mlst/index.html. Enterobacter cloacae isolates were left untyped as there is no established MLST protocol.
Conjugation experiments were performed between the clinical donor isolates and azide resistant Escherichia coli J53 as a recipient. Transconjugants were recovered from Luria-Bertani agar plates containing sodium azide (100 mg/L) and imipenem (5 mg/L) with PCR confirming the presence of blaNDM-1.
Fifty-two carbapenem non-susceptible isolates were screened with NDM-1 specific primers (Table 2). Of these, 12 isolates (23%) yielded a 291 bp amplicon, which upon sequencing, showed 100% identity with blaNDM-1 (GenBank:HQ162469). The 12 isolates positive for blaNDM-1 PCR were: six Klebsiella pneumoniae, four Escherichia coli and two Enterobacter cloacae. Full gene sequencing also confirmed that the genes encoded NDM-1 (Table 1). The 12 isolates were clearly Etest MBL (bioMérieux) positive. All the isolates were resistant to second- and third-generation cephalosporins and carbapenems (Table 3). Gentamicin resistance was seen in 9/12 (75%) of the isolates, with two isolates (16.7%) displaying a high level of amikacin resistance (Table 3). Resistance to chloramphenicol was also noted in 9/12 (75%) of the NDM-1 clinical isolates. High-level resistance to ciprofloxacin (≥32 mg/L) was seen in seven (58.3%) of the isolates. Susceptibility to tigecycline was retained by the NDM-1 positive isolates. Only one strain, Enterobacter cloacae isolate 459, was resistant to colistin, while the rest of the isolates retained their susceptibility (Table 3).

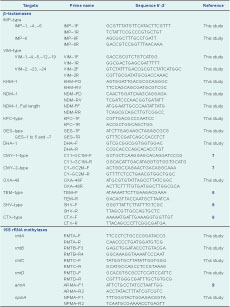
Note: AMK – amikacin; CAZ – ceftazadime;
CHL – chloramphenicol; CIP – ciprofloxacin;
COL – colistin; CTX – cefotaxime;
CXM – cefuroxime; ETP – ertapenem;
GEN – gentamicin; IMP – imipenem;
MEM – meropenem; PMB – polymyxin B ;
T – transconjugant; TET – tetracycline;
and TGC – tigecycline.
Five different sequence types were obtained for the six Klebsiella pneumoniae isolates. Two isolates with identical sequence type (ST 437) were isolated one month apart in separate wards. Isolate 547 and 594 derived from the same patient had dissimilar MLSTs. All the Escherichia coli isolates had differing sequence types. Hence, NDM-1 producing isolates comprised a variety of sequence types and were therefore genetically different (Table 1).
Conjugation experiments indicated that blaNDM-1 was transferable and likely via a plasmid-mediated event. A typical agarose gel electrophorectic profile of the plasmids analysed is shown in Figure 1. Plasmid content from clinical donor strains and transconjugants revealed that clinical NDM-1 isolates and their respective transconjugants carried a common band of covalently closed circular DNA larger than 28 kb in size (Figure 1). Southern hybridization analysis demonstrated localization of the NDM-1 gene to these plasmids (data not shown). We were unable to size the plasmids more accurately due to limitations by conventional gel electrophoresis.
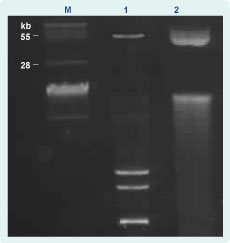
M - BAC-Tracker Supercoiled DNA Ladder (Epicentre); 1 - plasmid DNA from clinical isolate 380; and 2- plasmid DNA from 380 transconjugant.
All the isolates were PCR negative for MBLs and serine carbapenemase genes (OXA-48, KPC-1, GES-1, -2, -3, -4, -5 and -7). PCR results showed that it was not uncommon for the clinical NDM-1 producers to carry bla genes for more than one type of extended spectrum β-lactamases as well as plasmid-encoded AmpC β-lactamases (Table 1). These bla genes were transferable and conferred high levels of resistance to second- and third-generation cephalosporins and carbapenems to their recipient strain (Table 3).
Plasmid-mediated quinolone (qnr genes) and aminoglycoside resistance determinants (armA, rmtA, rmtB, rmtC, rmtD and npmA) were analysed by PCR. Enterobacter cloacae 459 was the only isolate found to be positive for qnrB. This determinant was not transferred to the transconjugant (Table 1). 16S rRNA methylase genes were detected in only one isolate, Enterobacter cloacae 241. In this isolate, armA was co-detected with rmtC (Table 1).
In this study, we observed differences from an initial report from Singapore regarding NDM-1 producers in which the NDM-1 positive isolates were likely to have been imported from India and Bangladesh.2 In contrast, the patients in our study had no recent travel history to countries where NDM-1 producers have been reported. The investigated isolates were obtained from four local hospitals and did not include the hospital of the initial report. Isolates from Tan Tock Seng Hospital were isolated over a four-month period, while the isolates at the National University Hospital were isolated over five months. No patient information was available for the isolates from the private hospital. Since the isolates originated from patients in differing wards and hospitals with their emergence being detected over a period of several months, we believe they were unlikely to have an epidemiological link. Epidemiological investigations done so far do not suggest nosocomial transmission of NDM-1 producing Enterobacteriaceae in these hospitals. However, we cannot completely rule out nosocomial transmission and further research is required to determine whether NDM-1 producing Enterobacteriaceae are endemic in Singapore.
The diversity of MLST strain types also indicates that the clones were genetically unrelated. Clonal diversity appears to be a characteristic of NDM-1 producers.12 This was reflected in a study looking at isolates of global origin, which revealed a large variety of strain types.12 The ease of dissemination of plasmid-bearing blaNDM-1 was apparent by the ability to obtain transconjugants for all the clinical donor strains. Similar findings on the ease of blaNDM-1 plasmid transmissibility have been noted.12,13 Investigations into the genetic context of blaNDM-1 reveal that the gene is frequently associated with insertion elements and often present on promiscuous plasmids bearing plasmid incompatibility groups Inc.A/C or non-typeable replicons.12,13 Due to the limited research capacity at our laboratory, we could perform only basic plasmid characterization. However, we acknowledge that PCR-based replicon typing14 is an important tool for epidemiologically tracing these blaNDM-1 plasmids.
Susceptibility profiling indicates low rates of colistin and tigecycline resistance in the NDM-1 producers and this appears to be a fairly typical observation among NDM-1 positive isolates.1,3 We note that aminoglycoside susceptibility is retained by most of the isolates. This finding differed from those of the Health Protection Agency, United Kingdom15 and Kumarasamy et al.,3 i.e. that most NDM-1 producers are typically aminoglycoside resistant and carry a 16S rRNA methylase gene. Only one of the isolates possessed 16S rRNA methylase genes (rmtC and armA), suggesting that aminoglycoside resistance arising in these NDM-1 positive isolates is mostly likely due to the more commonly encountered mechanisms of enzymatic inactivation mediated by acetyltransferases, nucleotidyltransferases and phosphotransferases.16 High-level quinolone resistance is mediated primarily by chromosomal mutations to the quinolone-resistance determining region in DNA gyrase.17 It is likely that the high levels of quinolone resistance seen in our isolates is mediated via this mechanism. Although we did not sequence the mutations of DNA gyrase gene of isolates, we did find plasmid-mediated quinolone resistance determinants, qnrB, in two isolates.
While it appears that there is no clonal outbreak of NDM-1 producing isolates in Singapore, the detection and dissemination of blaNDM-1 in the Asia Pacific region highlights the importance of surveillance efforts to understand more about these carbapenemase producers.
None declared.
This work was supported by a Health Service Development Programme Grant provided by the Ministry of Health, Singapore (Grant #HSDP06/X04) and a National University Health Systems grant.